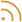Boarding Pass
NimBio unveils AI-powered Inflammometer to predict IBD flares before they strike
The company has raised a total of $2 million for its non-invasive technology, helping those with Inflammatory Bowel Diseases.
“For millions living with Inflammatory Bowel Diseases (IBD), such as Crohn’s disease and ulcerative colitis (UC), every flare is a painful reminder of how little control they have over their lives. Flare-ups are unpredictable, debilitating, and costly,” said NimBio. The company has identified a clear challenge: a lack of time and actionable alerts for chronic inflammatory conditions.
“But early detection changes everything: If we could spot inflammation earlier, we could reduce flare-related hospitalizations by up to 50%, saving an estimated $5 billion annually, even with conservative assumptions,” the company added.
1 View gallery


Professor Emeritus Yehuda Chowers, Co-founder and Chief Medical Officer,; Tal Atarot, Co-founder and CEO, NimBio
(Photo: NimBio)
NimBio has developed a non-invasive Inflammometer™ technology that can be part of everyday devices like smartwatches or fitness bands. With AI-powered, non-invasive technology, it can detect the early signs of inflammation in real time before a flare begins.
You can learn more about the company below.
Company Name: NimBio
Sector: Healthcare, AI
Product/Service description:
Inflammatory Bowel Diseases (IBD) strike without warning — at home, at work, in the middle of your life. Flares force emergency care, disrupt daily life, and drain quality of life. IBD leads to hundreds of thousands of hospitalizations each year — not because the disease is untreatable, but because we still can’t see the flare coming.
And the economic cost is just as serious: over €5 billion is spent every year in Europe alone, and nearly $30 billion in the US, much of it on hospitalizations and emergency care that could be prevented.
Despite advances in treatment, we still can’t predict when a flare will happen — and that’s the missing piece. Early flare detection is crucial for optimal treatment and results in improved disease control, reduced complication rates, and costs.
We’re changing that, with the world’s first non-invasive Inflammometer™.
Our vision for this technology is to be part of everyday devices like smartwatches or fitness bands. With AI-powered, non-invasive technology, it can detect the early signs of inflammation in real time before a flare begins.
It’s passive, comfortable, and personalized, no labs, no guesswork. And it gives patients and their doctors something they’ve never had before: time to act.
Founder Bios:
Tal Atarot B.Pharm, MSc - Co-founder and CEO: Pharmacologist and bioinformatician with 20 years of experience in developing medical technologies and managing clinical development across various diseases. He has held senior positions at the level of COO and VP in several startups, including Nutrinia, I2O Pharma, and Vecta. Among other things, he led clinical and regulatory operations with the FDA and EMA. He has extensive experience in developing medical technologies and leading global clinical and regulatory operations. In his recent roles, he led significant fundraising efforts, securing international funds.
Professor Emeritus Yehuda Chowers - Co-founder and Chief Medical Officer: Former Director of the Institute of Gastroenterology and the Research Division at Rambam Medical Center. Prof. Chowers specializes in inflammatory bowel diseases (IBD) and has been researching the immune system and its pathological manifestations for 26 years. He served as a member of the scientific committees of the European Crohn's and Colitis Organisation (ECCO) and United European Gastroenterology (UEG) and is considered a key opinion leader (KOL) in the field of IBD. His expertise in the pathophysiology of inflammatory diseases will assist in linking and understanding inflammatory variables relevant to the innovative index developed by NimBio.
Prof. Chowers also serves as CMO of CytoReason, a company specializing in computational models of biological and immunological data. Additionally, he has served on advisory boards for major pharmaceutical companies such as Pfizer, AbbVie, Janssen, Takeda, and Eli Lilly, which are key players in the field of IBD and other chronic inflammatory diseases.
Year of Founding: 2018
Last Investment Round: $500,000
Last Investment Stage: Pre-Seed
Date of Last Investment: January 2025
Total investment to date: $2 million
Investors: eHealth Ventures, Venturing Tech, Gigantum Investments, and angels Amnon Barl-Lev, Nir Kalkstein, and Ron Levkovich
Current number of employees: 4
How was the idea born?
The idea was born from a conversation with Professor Yehuda Chowers, a leading expert in IBD. Yehuda and Tal have known each other since 2006. They were discussing the persistent challenges patients face, especially the unpredictability of flare-ups and how current care is mostly reactive, along with the patient’s difficulties in adhering to biomarker testing (stool and blood, which would allow proactive care. We asked ourselves: What would it take to truly change the game for IBD patients and others living with chronic inflammatory conditions? That’s when it clicked: the holy grail isn’t just better drug treatment; it’s improving flare prediction, enabling early and timely intervention since the understanding that treating early is better was already obtained by others (the CALM study Colombel JF, et al. The Lancet. 2017;doi: 10.1016/S0140-6736(17)32641-7.) That insight became the foundation of NimBio.
What is the need for the product?
For millions living with Inflammatory Bowel Diseases (IBD), such as Crohn’s disease and ulcerative colitis (UC), every flare is a painful reminder of how little control they have over their lives. Flare-ups are unpredictable, debilitating, and costly.
The challenge is clear: for chronic inflammatory conditions, we still lack real-time, actionable alerts. That’s why flare-ups continue to lead to emergency room visits and unplanned hospitalizations. Not because it's untreatable, but because we can’t detect flare-ups early enough to intervene. The economic burden is equally alarming.
But early detection changes everything: If we could spot inflammation earlier, we could reduce flare-related hospitalizations by up to 50%, saving an estimated $5 billion annually, even with conservative assumptions. Beyond the cost savings, the human impact is even greater: better patient outcomes, fewer complications, and smarter, data-driven care.
Importantly, this isn’t just our vision — it’s the market’s demand.
The market is actively seeking solutions that shift chronic care from reactive to proactive, following the same continuous, data-informed model already standard in diseases like diabetes.
That’s why NimBio is gaining traction — and recognition:
Two Key Milestones:
- Strategic partnership following the EIT Open Innovation Challenge win
After winning the first prize in the EIT Open Innovation Challenge for IBD, NimBio signed a strategic Memorandum of Understanding (MoU) with Takeda (Belgium), KU Leuven, and EIT Health.
This alliance combines pharma scale-up expertise, a top-ranked IBD medical center, and Europe’s leading health innovation network to accelerate the NimBio solution for IBD through clinical validation, regulatory pathways, and European reimbursement readiness.
- Selection as an EIT Health Catapult 2025 finalist
NimBio’s selection as a Catapult finalist secured us a pitch slot at HLTH Europe 2025 in Amsterdam on June 17th, 2025 (happening next week!) and entry into EIT’s structured mentoring and due diligence program.
This visibility and vetting send a strong signal to investors and health systems and provide a “Stamp of Approval” for the innovation and market potential of the Nimbio product.
Together, these milestones show that NimBio is no longer just a promising technology — it’s an emerging solution positioned to predict flares, reduce hospitalizations, and transform care for IBD and beyond, all translated to a multi-million Euro market potential.
How is it changing the market?
NimBio is redefining how IBD — and potentially other chronic inflammatory diseases — are managed. We can help care providers, hospitals, and pharma companies prevent flares before they happen, using everyday wearables and a predictive AI platform to improve outcomes, reduce costs, and most importantly, give patients their lives back.
At the heart of this shift is the Inflammometer™. Unlike traditional wearables that rely on long-term monitoring, our solution requires only short bursts of passive data, making it personalized, more comfortable, scalable, and clinically actionable.
Here’s how it changes the game:
- Transforms invisible, unpredictable inflammation into actionable insight
- Turns wearables into medical-grade flare prediction tools
- Delivers real-time, personalized, proactive care with minimal burden on the patient
- Reduces hospitalizations, avoids complications, and optimizes healthcare resources
That’s what makes NimBio a platform shifting the chronic care model from reactive to predictive.
How big is the market for the product and who are its main customers?
NimBio is strategically positioned at the crossroads of two explosive markets:
The Chronic Inflammation Market, estimated at $130 billion with a projected CAGR of 8.5% from 2025 to 2030
The Digital Patient Monitoring Market, projected to reach $178 billion by 2030, growing at a CAGR of 25.2%
Together, these markets represent an enormous opportunity for scalable impact, both in terms of revenue potential and global health outcomes.
NimBio’s solution is tailored for:
- Hospitals and IBD Clinics: For flare detection as a clinical decision-support tool — reducing ER visits and inpatient costs
- Pharma Companies: For real-time patient monitoring in trials, flare prediction in companion biomarkers, and value-based care partnerships
- Payers and Health Systems: To drive down chronic care costs and avoid unnecessary hospitalizations
- Digital Health Platforms: To integrate predictive IBD monitoring into chronic condition management platforms
Does the product exist already? If not - at what stage is it, and when is it expected to hit the market?
The core technology is already functional. We’re currently collaborating with a U.S.-based wearable vendor as our hardware providers while we focus on the algorithms and software platform. In parallel, we are moving through a structured regulatory path to hit the market in 2026.
Our technology is:
- Patent-protected
- Under ongoing clinical validation
We're working directly with pharma companies and top-ranked IBD centers
By combining wearable data and predictive AI, NimBio is shaping a new category of non-invasive, real-time inflammation monitoring.
Who are the main competitors in this sector and how big are they?
In the realm of IBD management, NimBio competes with traditional tools and legacy approaches that require active patient participation, such as:
- Stool sample collection for calprotectin measurement
- Blood tests through HMOs or clinics
- Symptom tracking questionnaires and manual reporting
These methods not only disrupt patients’ daily lives, but also suffer from low compliance and inconsistent data. Even digital solutions built on these models still depend on manual inputs or frequent lab access.
What is the added value that the founders bring to the company and the product?
At NimBio, the founders bring a unique combination of scientific expertise, entrepreneurial experience, and deep industry networks, which is critical in solving a challenge as complex as IBD flare prediction.
Co-founder Professor Yehuda Chowers is a globally recognized expert in IBD with over two decades of experience treating patients, running clinical trials, and publishing groundbreaking research. His insight ensures our technology is not just innovative — it's clinically relevant, evidence-based, and aligned with real-world care workflows.
Co-founder Tal Atarot brings over 18 years of experience leading ventures across health innovation and medtech, with a proven track record in building cross-disciplinary teams, navigating regulatory strategy, and securing partnerships.
In addition to the team’s expertise, NimBio is backed by some of the most respected health innovation players:
- eHealth Ventures – Israel’s leading HealthTech VC
- The Israel Innovation Authority
- VenturingTech (EU-based investor) and other European investors
Together, the founders and our strategic investors give NimBio the clinical insight, operational strength, and network reach to turn innovation into impact
What will the money coming in from the round be used for?
The funds from this round will be used to drive regulatory approval and prepare for market entry. Our top priority is conducting a regulatory study in collaboration with our European consortium partners, paving the way to obtain a CE mark for our solution and enabling its market entry during 2026.
In the "Startup Boarding Pass" section, CTech will cover the (relatively) small investments made in companies during the early stages of their existence - and the entrepreneurs and startups who have not yet had the opportunity to reveal their stories to the world. Please use the linked form and fill it out according to the guidelines. This form is intended for startups raising between $500,000 and $3 million from venture capital funds, angels, or official grants from Israeli and foreign institutions. If relevant, someone at CTech will be in touch for follow-up questions.